Written By: Dr. Stephen Campbell, PhD, CSci, CChem, MRSC, Chief Technology Officer at Nano One Materials Corp.
The drive for energy storage solutions for transportation and renewable energy grid storage signals a major shift away from fossil fuels towards an environmentally sustainable future. This depends, to a large extent, on the development of effective and affordable lithium-ion batteries (LiB) that must deliver high energy storage capacity and long lifetime without being prohibitively expensive. The most widely recognized cathode active materials used in LiBs today are lithiated transition metal oxides containing lithium (Li), nickel (Ni), manganese (Mn) and cobalt (Co), known as NMC and to a lesser extent lithium iron phosphate, containing lithium (Li), iron (Fe) and phosphorous (P), known as LFP. Recent price volatility and supply chain risk has caused battery manufacturers to give greater consideration to iron-based cathode materials because the raw material supply is less constrained, less costly and the resulting cathode is very safe and durable. Demonstrated in 1996 by Nobel Laureate John Goodenough, lithium iron phosphate, LFP or LiFePO4 is set to become a major component, alongside NMC in the future of energy storage.
What is LFP and how is it different from NMC?
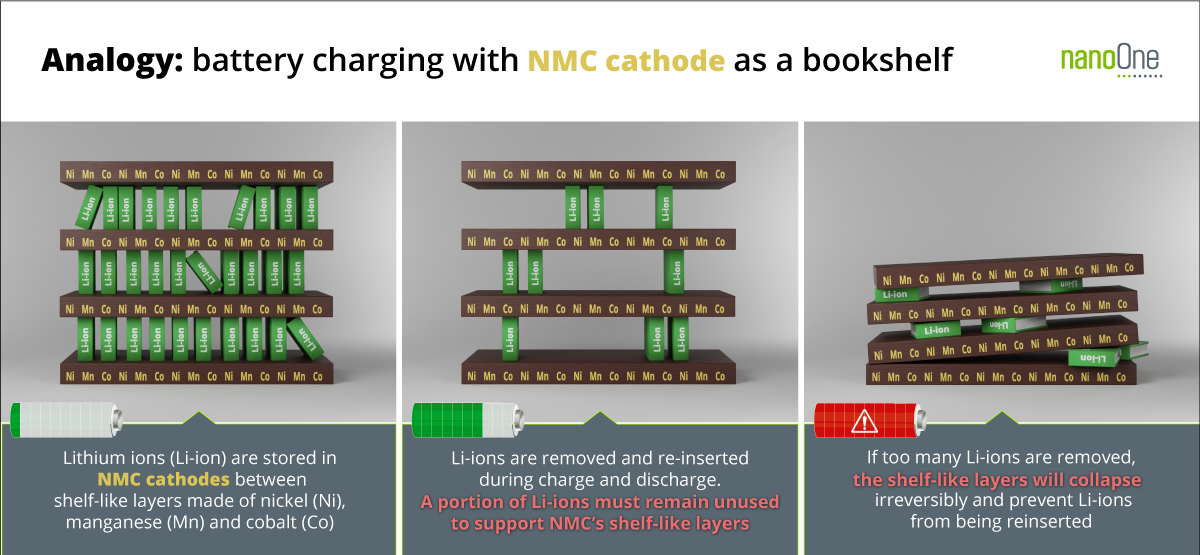
NMC materials are metal oxides that incorporate lithium (Li) into the crystal structure, forming layers of transition metals with space between the layers for the Li ions to move in and out as the cell is discharged and charged. If all the Li is removed the NMC structure will collapse and stop working thereby limiting the amount of charge available to the cell and leaving a portion of the Li, Ni, Co and Mn unused with diminished efficiency and sustainability. These materials are not very stable and abuse can lead to loss of oxygen causing a potential fire risk. (See Figure 1 for Analogy: battery charging with NMC cathode as a bookshelf)

Figure 1. In NMC based lithium-ion batteries, if too many lithium ions (Li-ion) are removed during charging NMC’s bookshelf-like layers will collapse and prevent Li-ions from being re-inserted during discharge. This limits the utility, efficiency and sustainability of lithium, nickel (Ni), manganese (Mn) and cobalt (Co) battery metals.
LFP is an olivine crystal structure in which the iron is bound to a phosphate ion (PO43-) which is known to be chemically very stable, and as such can be readily found in nature; the P-O bond is very strong meaning that oxygen loss, and risk of fire, is very unlikely. Another unique attribute of LFP olivines is that all of the Li may be removed and re-inserted without collapsing the structure for maximum charge and discharge of the cell. LFP uses all of Li, Fe and P in the cell, for maximum efficiency and sustainability and this is contrasted with NMC in Figure 1. The lack of oxygen loss and structural change means that LFP cathode materials are very stable and cycle lifetime of many thousand cycles are achievable. (See Figure 2 Analogy: battery charging with LFP cathode as a bookshelf)

Figure 2. In LFP based lithium-ion batteries, all of the lithium ions (Li-ions) may be removed and re-inserted during charging and discharging without collapsing LFP’s cupboard-like compartments. This maximizes the utility, efficiency and sustainability of the lithium, iron (Fe) and phosphorus (P) battery metals.
One of the difficulties with LFP is that it is not as conductive as the oxides like NMC. Because the material must serve as an electrode, electrical conductivity is critical for its operation in a cell. It was discovered that if the individual crystals of LFP were coated in conductive carbon, LFP would function very well in a cathode and, although methods such as doping with other metals was investigated, carbon coated LFP has become the standard for this type of cathode.
The theoretical charge capacity of LFP is 170mAh/g and practical capacities of 120 – 160mAh/g are common depending upon the application. The discharge cell voltage is around 3.2V. These values are lower than conventional NMC cathodes but offset with greatly improved durability, safety and battery pack energy densities made possible by the chemical stability of the phosphate. LFP full cells may survive up to 9,000 charge/ discharge cycles, which is currently unparalleled in LiBs (Sandia National Laboratory, 2020)1. The long life and extra durability greatly enhances the economics, efficiency and sustainability of LFP batteries.
The cost of Fe remains low and stable compared to the extreme market volatility of Ni and Co. Unlike Ni and Co, the global markets for Fe and P dwarf LiB demand projections, making security of supply in the long-term much greater for LFP than NMC. This means that the cost of LFP should be considerably lower than Ni and Co laden NMC, particularly when considering cycle life and total cost of ownership over the lifetime of the battery ($ per kWh per cycle). The form of lithium used to make LFP is also more flexible because lithium hydroxide and carbonate may both be used. Ni-rich NMC currently requires lithium hydroxide for manufacture, although companies like Nano One have developed methods that use lithium carbonate as well. This means that the cost and availability of LFP compares very favourably when compared to NMC cathode materials.
NMC will remain of great importance for long range electric vehicle applications, and LFP will be better suited to heavier duty cost-sensitive applications in industry, renewable energy storage and mass market low and mid-range urban vehicles. Nano One has developed a novel process that is applicable to both NMC, LFP and other cathode materials, reducing complexity, cost and environmental footprint. For making LFP, it uses a supply chain that is more competitive and better suited to markets outside of China. This technology is in the process of being scaled up towards commercialization for applications in North America, Europe and other emerging jurisdictions .
In summary, LFP may grow to have significant market share, alongside NMC, for both EVs and renewable energy storage. LFP is lower cost, longer lasting and safer, which offsets its lower energy density. Together with recent innovations in the energy density of LFP cells and battery packs, electric vehicle and renewable energy storage manufacturers are able to meet the range and performance requirements of the future.
[1] Yuliya Preger et al; J. Electrochem. Soc. 167 (2020) 120532